PLINABULIN
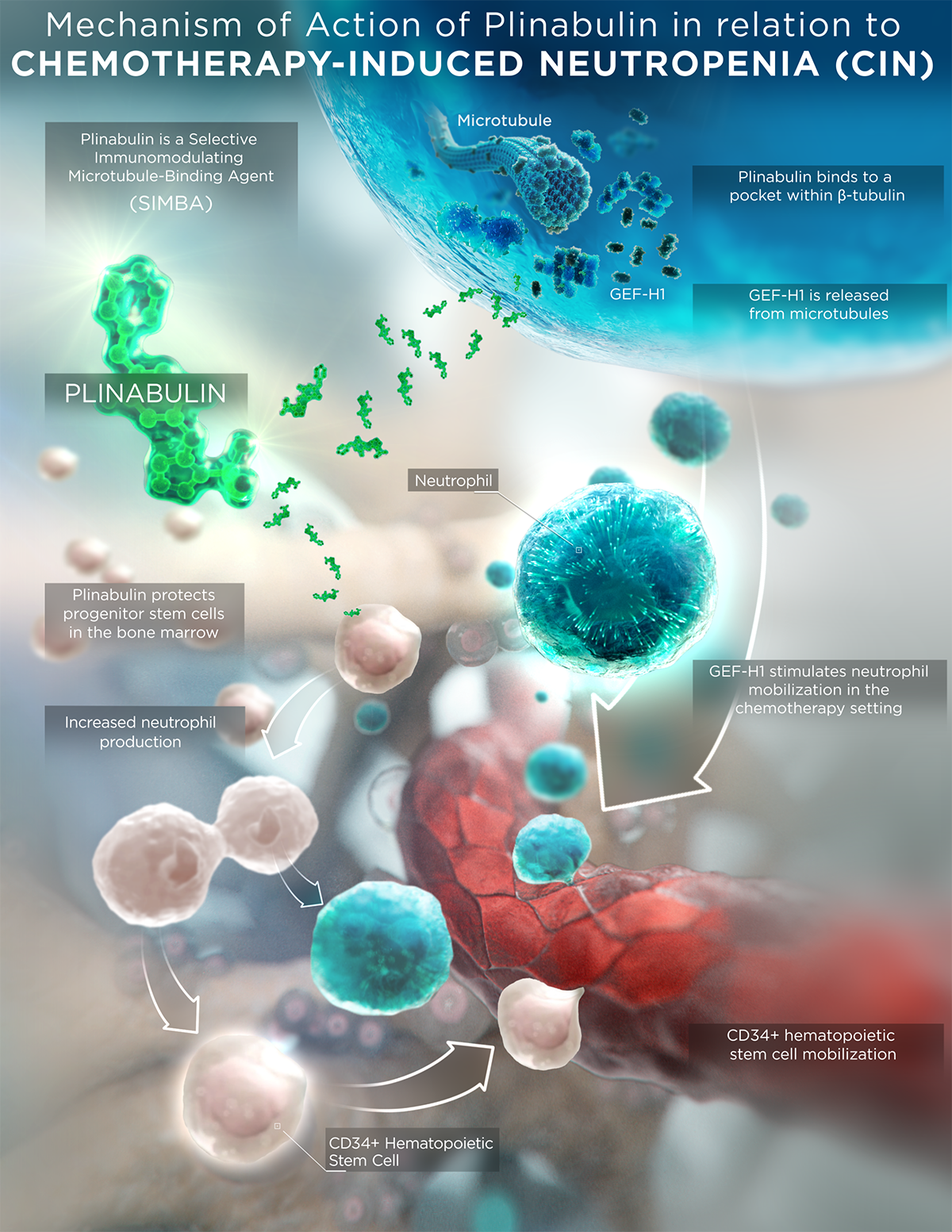
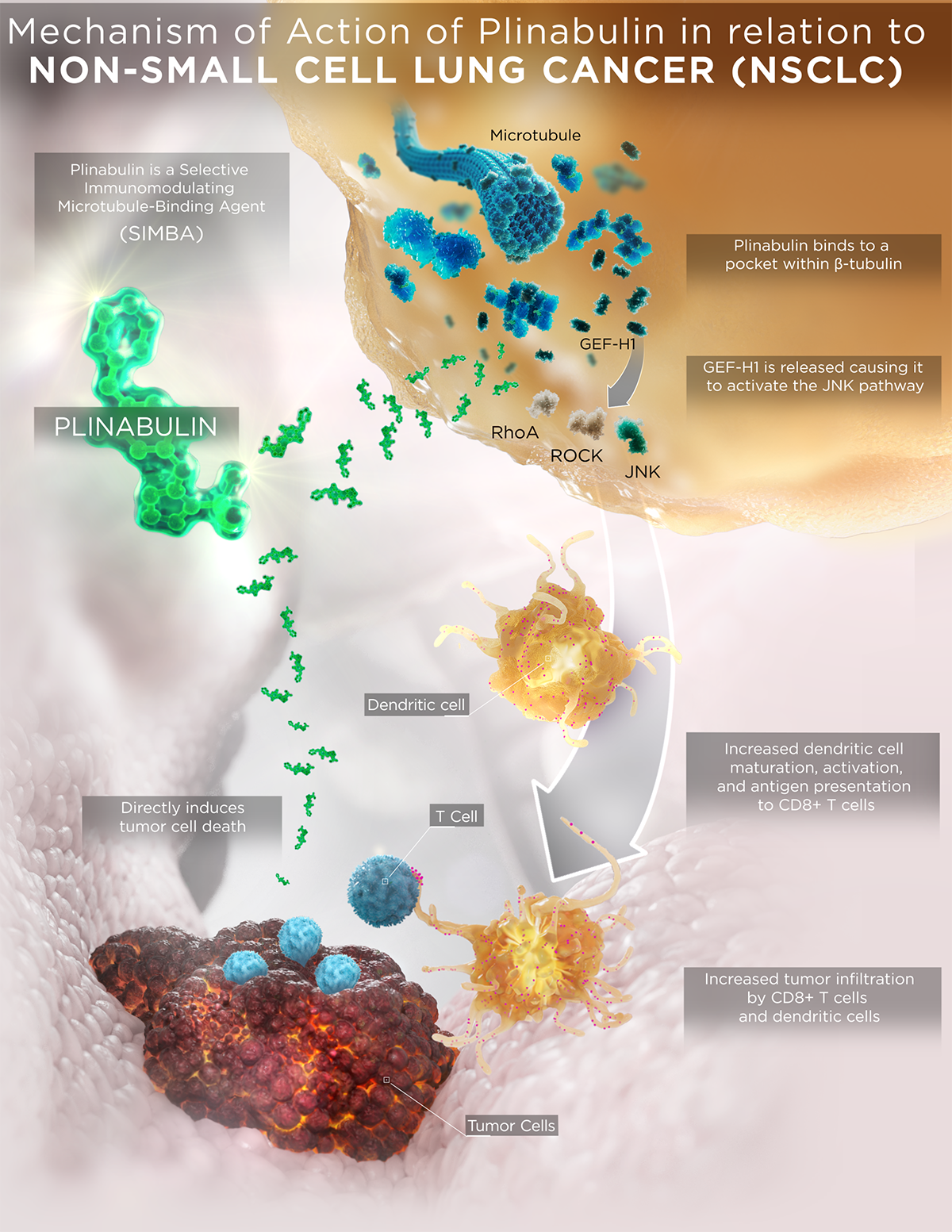
Plinabulin is a first-in-class selective immunomodulating microtubule-binding agent (SIMBA) with potential chemotherapy-induced neutropenia (CIN) prevention and anticancer benefits.
Plinabulin is investigational and not FDA approved.
PROPOSED MOA
Plinabulin is an investigational small molecule that is in the new class of selective immunomodulating microtubule-binding agents. It features a unique mechanism of action (MOA) compared to current standard-of-care chemotherapeutics and conventional microtubule-binding agents, which result in differentiated safety and efficacy profiles.
Plinabulin binds in a differentiated pocket of tubulin with different binding kinetics from other tubulin binding agents, including taxanes, vincas, and colchicine (La Sala Chem 2019). This differentiation in binding not only results in a well tolerated safety profile in >700 cancer patients, but also in differentiated immune-modulating biological activities for plinabulin.
PLYNRESCA™
- Plinabulin triggers the release of the immune defense protein, GEF-H1, which leads to a durable anticancer benefit due to the maturation of dendritic cells (antigen resenting cell (APC)) resulting in the activation of tumor antigen-specific T-cells to target cancer cells (Kashyap Cell Reports 2019).
- Plinabulin also exerts an early-onset of action in CIN prevention in week 1 after chemotherapy by boosting the number of hematopoietic stem/progenitor cells (HSPCs) (Tonra Cancer Cgemother Pharmacol 2020).
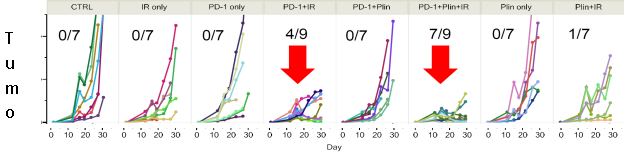
In PD-1 non-responsive immune competent animal model, Plinabulin in combination with a PD-1 antibody and radiation (IR) – triple IO combination – induces approximately 80% of tumor reduction, almost double the response rate in the PD-1 antibody and radiation.
Increased DC maturation through MHCII upregulation, and T cell activation in triple IO combination with plinabulin were observed in tumor samples 30 days after drug use, compared to the PD-1 and radiation arm.
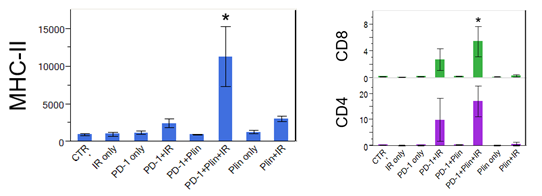
*Neri S. et al. Plinabulin, a microtubule destabilizing agent, improves tumor control by enhancing dendritic cell maturation and CD8 T cell infiltration in combination with immunoradiotherapy, AACR June 2020
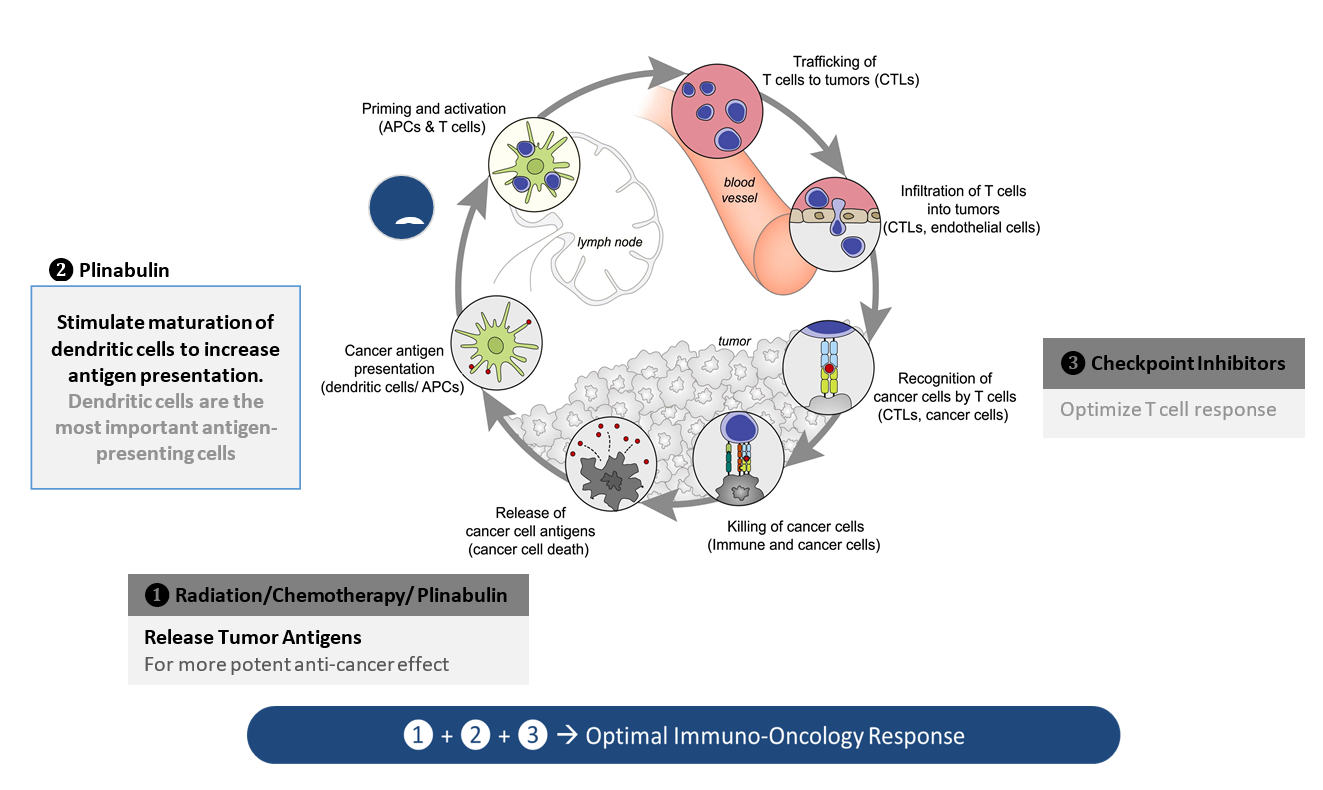
With these unique MOAs, Plinabulin is being developed as a “pipeline in a drug”, in a number of cancer indications as a direct anti-cancer agent in NSCLC, and in combination with immuno-oncology regimens, as well as to prevent chemotherapy-induced neutropenia (CIN).
- In the Phase 3 DUBLIN-3 study, the plinabulin and docetaxel combination met the primary endpoint of extending overall survival compared to docetaxel alone in 2nd/3rdline NSCLC (EGFR wild type).
- In Phase 1 IIT Big Ten Study, plinabulin combined with PD-1 and CTLA-4 antibodies had ORR of 43% in 2nd/3rd line SCLC who failed platinum and PD-1/PD-L1 antibodies.
- In addition, plinabulin received Breakthrough Therapy designation from both U.S. and China FDA for the CIN prevention indication.
MOA video animation
Plinabulin is investigational and not FDA approved.

The highly differentiated MOA is published in numerous industry journals, including Chem and Cell Reports. Click a publication below to read the full article.
CHEM
Structure, Thermodynamics, and Kinetics of Plinabulin Binding to Two Tubulin Isotypes

CELL REPORTS
GEF-H1 Signaling upon Microtubule Destabilization is Required for Dendritic Cell Activation and Specific Anti-tumor Responses

NSCLC
Second- and third-line NSCLC with EGFR wild type patients are patients with severely unmet medical needs. As PD‑1/PD-L1 inhibitors have moved to first line, docetaxel and docetaxel-based regimens have become the standard of care in 2nd/3rd line, with an approximately 10% 2‑year survival rate, and around a 5% 3‑year survival rate and >40% severe neutropenia, which needs improvement. Once patients fail a CPI regimen in first line, they are no longer candidates for CPI in 2nd/3rd line.
For 2nd/3rd line NSCLC with EGFR mutant patients, median OS is around 18 months treated with TKI (Ramalingam et al, 2020). While for EGFR wild type patients, the median OS is 5.4 months treated with TKI (e.g. Tarceva), which is worse than the median OS at 8.2 months when treated with docetaxel in the TAILOR study (Garassino et al, 2013).
Current SOC 2nd/3rd Line Therapies for NSCLC, EGFR Wild Type
| Efficacy | Safety | |
|
Docetaxel (75 mg/m2) (Fosella et al, 2000) |
Median OS: 5.7 months (M) ORR: 6.7% 1‑year OS rate: 32% |
Severe neutropenia: 54-77% |
|
Ramucirumab + Docetaxel vs. Docetaxel (Garon et al, 2014) |
Median OS: 10.5 M (combo) vs. 9.1 M (docetaxel); HR=0.86 ORR: 23% (combo) vs. 14% (docetaxel) Median PFS: 4.5 M (combo) vs. 3 M (docetaxel), HR=0.76 |
Severe neutropenia: 49% (combo) vs. 40% (docetaxel) Bleeding or hemorrhage events: any grade 29% (combo) vs. 15% (docetaxel) |
|
Pemetrexed vs. Docetaxel (Hanna et al, 2004) |
Median OS: 8.3 M (pemetrexed) vs. 7.9 M (docetaxel), HR=0.99 ORR: 9.1% (pemetrexed) vs. 8.8% (docetaxel) Median PFS: 2.9 M (pemetrexed) vs. 2.9 M (docetaxel), HR=0.97 |
Severe neutropenia 5.3% (pemetrexed) vs. 40.2% (docetaxel) |
Abbreviations: EGFR: epidermal growth factor receptor; HR: hazard ratio; M: months; NSCLC: non‑small cell lung cancer; ORR: objective response rate; OS: overall survival; PFS: progression free survival
CLINICAL TRIALS
For details of these two trials, please visit below websites.
PROGRAMMED CELL DEATH PROTEIN 1 (PD-1) COMBINATION
PLINABULIN + PD-1 + CTLA-4 Antibodies
In October 2018, we announced the opening of an investigator-initiated phase 1 clinical trial with a triple combination therapy, consisting of plinabulin, nivolumab, and ipilimumab, for the treatment of 2nd and 3rd line small cell lung cancer.
The trial, conducted through the Big Ten Cancer Research Consortium, which includes Rutgers Cancer Institute of New Jersey and other clinical centers in the U.S.. This study investigates whether the addition of plinabulin results in a reduction of immune-related side effects of PD-1 and cytotoxic T-lymphocyte-associated protein 4 antibodies and if it provides efficacy synergy. Phase 1 had completed and phase 2 study had initiated in September 2021.
Study phase 1 Dose-escalation phase I study 3+3 Design
In patients with extensive-stage SCLC who had progressed on or after prior platinum-based chemotherapy (±PD-1/PD-L1)
Primary objective
To determine dose-limiting toxicities (DLT’s) and recommended Phase 2 dose (RP2D).
Patients received treatment until progression or intolerable toxicity.
Patients were evaluable for DLT if they received at least 2 cycles of therapy
DLT period was defined as the first 6 weeks from C1D1.
Secondary endpoints:
ORR, PFS
Frequency of Ir-AEs.
Phase 1 study was completed with promising data and was presented at ASCO 2021
PLINABULIN + PD-1/PROGRAMMED DEATH LIGAND 1 (PD-L1) + RADIATION/CHEMO
In July 2018, we entered into a sponsored research agreement with MD Anderson Cancer Center to evaluate the benefits of adding plinabulin to radiation therapy plus immune checkpoint antibodies. The study has demonstrated that this triple combination approach (plinabulin + radiation + PD-1 antibody) has dramatic benefits in tumor reduction, increasing tumor dendritic cell maturation and increasing tumor T-cell infiltration (AACR 2020 poster link).
Given the high incidence of progression with PD-1/PD-L1 antibody therapies in the majority of cancers, we believe this novel triple combination approach will restore or enable the immune targeting of cancer in patients that have progressed on checkpoint-targeted therapy. The phase 1/2 has initiated at MD Anderson and first patient was enrolled in June 2021. Extensive immune biomarker analysis is conducted in this IIT study.
CLINICAL TRIALS
For details of these two trials, please visit below websites.
CIN
Plinabulin is being evaluated for the prevention of CIN and is in two global multi-center clinical trials aiming for a broad indication of prevention of all CIN in all non-myeloid cancer types.
PROTECTIVE-1 (Study 105) is a phase 2/3 trial of plinabulin versus Neulasta after a standard regimen of docetaxel, an intermediate-risk chemotherapy, for advanced breast cancer, hormone-refractory prostate cancer and advanced NSCLC patients.
PROTECTIVE-2 (Study 106) is a phase 2/3 trial of plinabulin in combination with Neulasta, a long-lasting granulocyte-colony stimulating factor (G-CSF), (plinabulin/Neulasta combination) versus plinabulin monotherapy versus Neulasta monotherapy after a high risk myelosuppressive chemotherapeutic regimen composed of three agents, Taxotere (docetaxel), Adriamycin (doxorubicin) and Cytoxan (cyclophosphamide), or TAC, for patients with advanced breast cancer.
We believe that the clinical profile of plinabulin in combination of a G-CSF (plinabulin/G-CSF) such as its potential for improved clinical outcome in neutropenia, lower bone pain, prevention of immune suppression, and its anticancer effects, has the potential to set a new standard in the prevention of CIN in patients treated by high-risk chemotherapy. By improving clinical outcomes in neutropenia and reducing bone pain, the plinabulin/G-CSF combination has the potential to enable patients to maintain chemotherapy target dose, keep to their chemotherapy cycle times, and complete the full course of chemotherapy. When this occurs, patients have significantly better long-term outcomes.
Plinabulin is an investigational and first-in-class selective immunomodulating microtubule-binding agent (SIMBA) currently being studied in clinical trials (NCT03294577; NCT03102606) for concurrent administration with myelosuppressive chemotherapeutic regimens in patients with solid (non-myeloid) cancers for the prevention of CIN and may potentially show beneficial effects in lowering bone pain, preventing immune suppression, and reducing thrombocytopenia in patients treated by intermediate-risk chemotherapy where G-CSF is not recommended.
CLINICAL TRIALS
For details of these two trials, please visit below websites.
TRIPLE COMBINATIONS
PLINABULIN + NIVOLUMAB + IPILIMUMAB
In October 2018, we announced the opening of an investigator-initiated phase 1 clinical trial with a triple combination therapy, consisting of plinabulin, nivolumab, and ipilimumab, for the treatment of small cell lung cancer. The trial, conducted through the Big Ten Cancer Research Consortium, is currently enrolling subjects at Rutgers Cancer Institute of New Jersey and other clinical centers in the U.S. This study investigates whether the addition of plinabulin results in a reduction of immune-related side effects of PD-1 and cytotoxic T-lymphocyte-associated protein 4 antibodies and if it provides efficacy synergy.
PLINABULIN + PD-1/PROGRAMMED DEATH LIGAND 1 (PD-L1) + RADIATION/CHEMO
In July 2018, we entered into a sponsored research agreement with MD Anderson to evaluate the benefits of adding plinabulin to radiation therapy plus immune checkpoint antibodies. The study has demonstrated that this triple combination approach (plinabulin + radiation + PD-1 antibody) has dramatic benefits in tumor reduction, increasing tumor dendritic cell maturation and increasing tumor T-cell infiltration. Given the high incidence of progression with PD-1/PD-L1 antibody therapies in the majority of cancers, we believe this novel triple combination approach will restore or enable the immune targeting of cancer in patients that have progressed on checkpoint-targeted therapy.
CLINICAL TRIALS
For details of these two trials, please visit below websites.