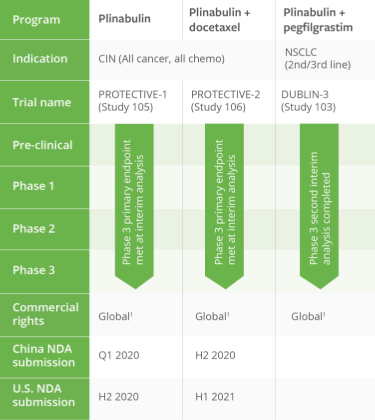
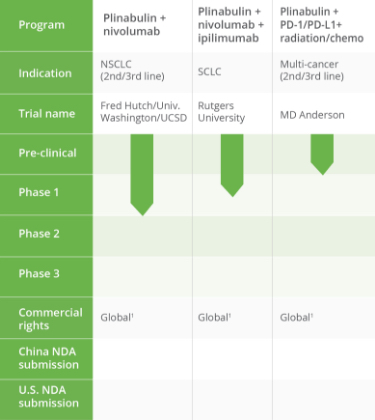
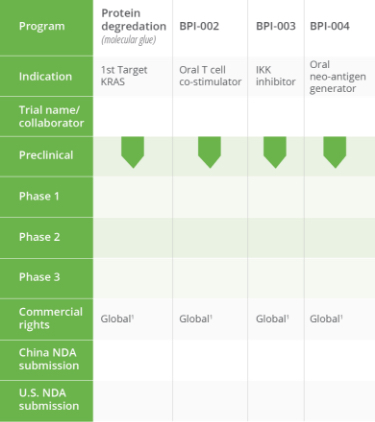
PIPELINE OVERVIEW
We have a robust drug development pipeline at various clinical and preclinical stages.
Tap the sections below to expand or download the full pipeline chart.
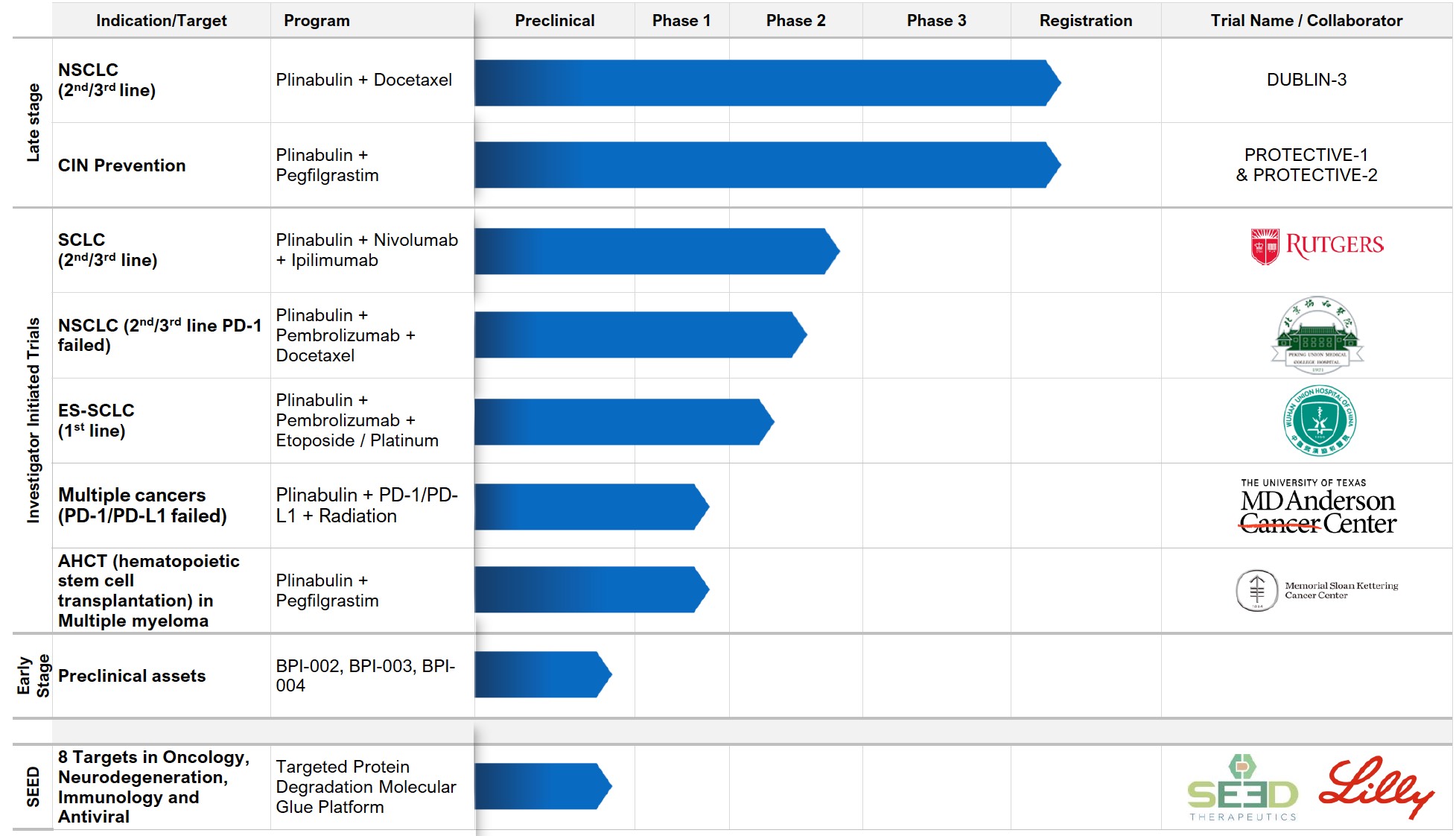
BeyondSpring Subsidiaries: 1) Dalian Wanchunbulin Pharmaceuticals Ltd., which owns China rights to Plinabulin, and 2) Seed Therapeutics, a target protein degradation company.